
Recently, the team of Professor Yang Yi and Academician Bian Xiuwu from the First Affiliated Hospital of Army Medical University, Jinfeng Laboratory, and Professor Qi Xiaowei's team published a research paper titled "Tumor PD-L1 induces β2m ubiquitylation and degradation for cancer cell immune evasion" in the Cell Research journal. This study discovered for the first time that PD-L1 in the well-known immune checkpoint signaling pathway PD-1/PD-L1 actually also has E3 ubiquitin ligase activity. Specifically, PD-L1 degrades β2m, an important component of the antigen-presenting protein MHC-I, through ubiquitination, leading to a reduction in the level of MHC-I on the surface of cancer cells, thereby helping cancer cells avoid recognition by CD8-positive T cells and driving tumors to become resistant to immune checkpoint inhibitors. This discovery reveals an unprecedented new function of PD-L1 in tumor cell immune evasion and expands our understanding of the intrinsic resistance mechanism of immune checkpoint blockade (ICB) therapy.

At the beginning of the study, the researchers originally wanted to find the E3 ubiquitin ligase that regulates PD-L1 expression levels. Surprisingly, PD-L1 was ubiquitinated in the absence of the selected E3 ubiquitin ligase.
After excluding the influence of other E3 ubiquitin ligases, the researchers determined that PD-L1 can spontaneously ubiquitinate. In other words, PD-L1 has the activity of E3 ubiquitin ligase, but PD-L1 lacks the typical E3 ubiquitin ligase domain (this may also be the reason why this function has only been discovered so far). They also confirmed that the ubiquitination process of PD-L1 occurs in the endoplasmic reticulum of cells and that the E3 ubiquitin ligase activity of PD-L1 does not depend on binding to PD-1.
Since PD-L1 has E3 ubiquitin ligase activity, the biological function of this activity must be studied. Based on different types of gene knockout/mutated cancer cell lines, researchers found that in addition to inhibiting T cell anti-tumor activity through the PD-1/PD-L1 pathway, the E3 ubiquitin ligase activity of PD-L1 can also promote tumor immune evasion, and the latter is related to the antigen presentation protein MHC-I.
So who is the substrate for PD-L1 ubiquitination? Research at the proteomics level has found that β2m, which is involved in MHC-I antigen processing and presentation, can be directly ubiquitinated by PD-L1. Studies in in vitro cell lines show increased stability of β2m in the absence of PD-L1 ; In the case of PD-L1 overexpression, β2m degradation is accelerated, and β2m mRNA levels are not affected.
Since β2m is critical for the cell surface level and stability of the antigen processing/presentation protein MHC-I, the E3 ubiquitin ligase activity of PD-L1 is bound to affect MHC-I. This is indeed the case. After PD-L1 induces ubiquitination and degradation of β2m, it will lead to a decrease in MHC-I levels on the surface of cancer cells and antigen-presenting cells, thereby inhibiting anti-tumor immunity.
Based on clinical samples from breast cancer and lung cancer patients, the researchers found a significant negative correlation between PD-L1 and β2m protein levels. This finding is also consistent with the above-mentioned research results. From this point of view, in clinical treatment, targeting the PD-L1-mediated β2m ubiquitination pathway may be a therapeutic strategy to enhance the efficacy of PD-L1/PD-1 inhibitors.
By introducing mutations in PD-L1 to eliminate the E3 ubiquitin ligase activity of PD-L1 or disrupt the interaction between PD-L1 and β2m, the researchers further confirmed that the E3 ubiquitin ligase activity of PD-L1 promotes immune escape and limits immunotherapy response by driving β2m ubiquitination and degradation. They also confirmed that the above two ways of inhibiting β2m ubiquitination significantly enhance the sensitivity of tumors to PD-L1 inhibitors.
At the end of the study, the researchers looked at clinical data from triple-negative breast cancer patients treated with PD-1 inhibitors and found that patients who did not respond to immunotherapy had significantly lower β2m expression levels than responders. Based on this, they believe that when the β2m gene copy number or expression level is high enough, PD-L1-mediated degradation has little effect on the total β2m protein level. ; In patients with relatively low basal β2m expression, PD-L1-driven ubiquitination and degradation of β2m will lead to reduced MHC-I expression and ultimately lead to tumor resistance to immune checkpoint inhibitors.
Overall, the results of this study indicate that PD-L1 not only exerts an immunosuppressive effect through the classic immunosuppressive signaling pathway PD-1/PD-L1, but also reduces MHC-I levels through its E3 ubiquitin ligase activity, helping tumors achieve immune escape. The results of this study also suggest that when the basal expression level of β2m is low, blocking the PD-L1-PD-1 interaction alone is not enough to effectively inhibit tumor growth. Therefore, β2m may be used as a biomarker to predict the effect of immunotherapy. In addition, targeting the E3 ubiquitin ligase activity of PD-L1 may be a way to improve the therapeutic effect of PD-L1/PD-1 inhibitors in patients with low β2m expression.
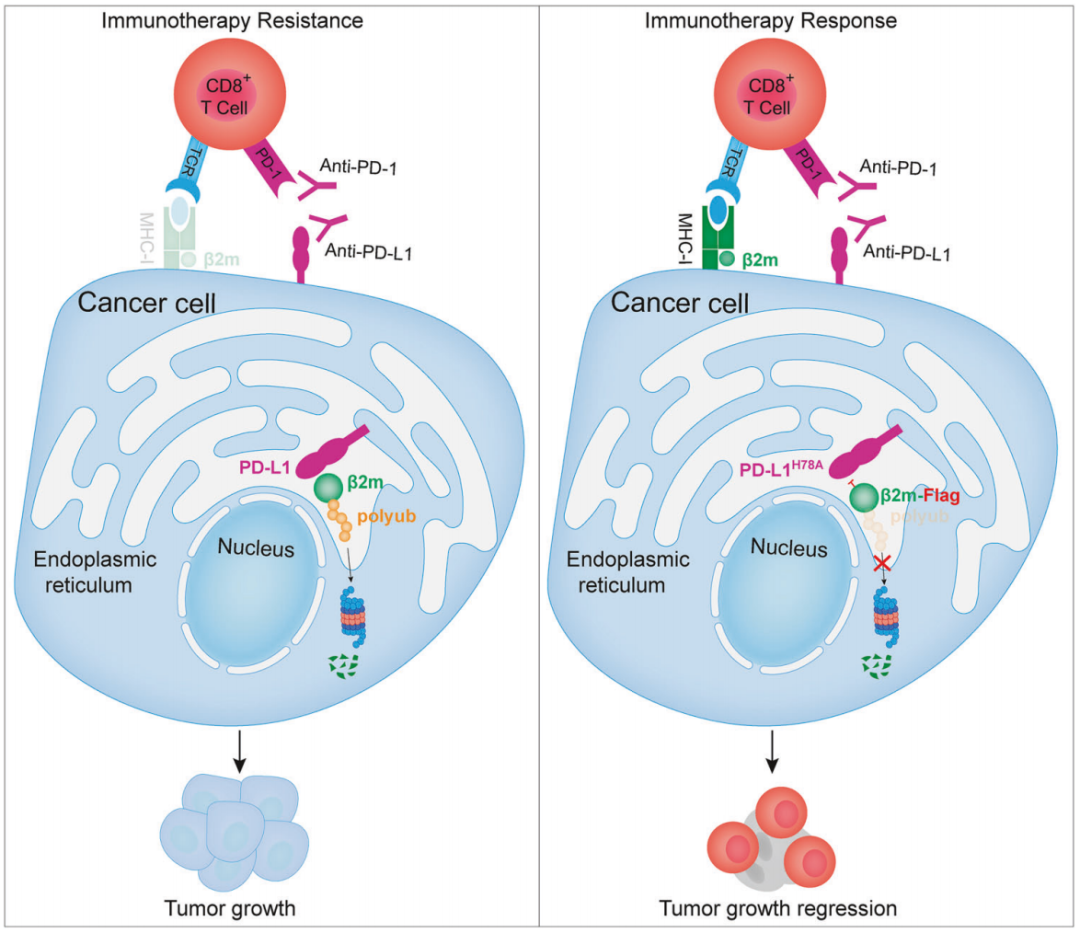
▲The role of PD-L1-mediated β2m ubiquitination in tumor immune evasion
"Cell Research" was founded in 1990. It is an international academic journal managed by the Chinese Academy of Sciences, co-sponsored by the Center for Excellence in Molecular Cell Science of the Chinese Academy of Sciences and the Chinese Society of Cell Biology.(Impact factor25.9,JCR Q1 district), Published in cooperation with Springer Nature, it mainly publishes outstanding original research papers, newsletters, reviews and authoritative reviews in the field of life sciences. It is the broad-spectrum journal in the field of life sciences with the highest impact factor in China and even Asia.
Click on the lower left to read the original text and access the original text link.