
Recently, the team of Professor Liang Li from Southern Medical University and Jinfeng Laboratory published a research article titled Uridine Depletion Impairs CD8⁺ T Cell Antitumor Activity through N-Glycosylation in the journal Cell Metabolism. This study revealed that uridine mediates N-glycosylation of CD45 protein to promote CD8⁺ The new mechanism of T cell anti-tumor immunity systematically elaborates on the formation process and regulatory mechanism of uridine depletion in the tumor microenvironment, and proposes new targets and combination treatment strategies for the diagnosis and treatment of immunotherapy resistance.

Through multi-omics data mining and clinical cohort analysis, this study found that SNX17 expression was negatively correlated with CD8⁺T infiltration in tumors and ICB treatment sensitivity. Mechanistically, SNX17 regulates the metabolic microenvironment by competing for the uptake of uridine, leading to uridine depletion, thereby inhibiting CD8⁺ T cell function and triggering resistance to PD-1 antibody treatment. Uridine supplementation reversed this suppression and inhibited tumor growth.
Uridine activates CD8⁺ T cells by promoting N-glycosylation of CD45. UDP-GlcNAc produced by uridine metabolism can enhance CD45 glycosylation, thereby promoting LCK/ZAP70 phosphorylation and strengthening TCR signaling. Inhibiting N-glycosylation or CD45 function blocks this activation.
Upstream mechanism studies have shown that SNX17 prevents lysosomal degradation by directly binding to and stabilizing the transcription factor RUNX2. Stable RUNX2 then transcriptionally upregulates the expression of the uridine-degrading enzyme UPP1, accelerating the decomposition and consumption of uridine, ultimately leading to a lack of uridine in the tumor microenvironment.
In summary, This study found that uridine is a modifiable CD8⁺ T Cell-active immunometabolites reveal the molecular mechanism by which cancer cells inhibit anti-tumor immunity by reducing uridine levels in the tumor microenvironment; SNX17 Uridine may serve as a predictive biomarker for resistance to cancer immunotherapy, and uridine may be a promising immunotherapy drug.
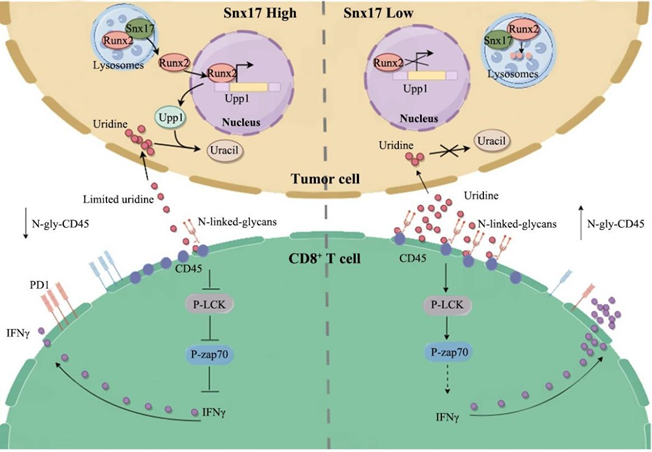
▲Schematic diagram of the mechanism of uridine regulating CD8⁺ T cell anti-tumor immunity.
"Cell Metabolism" was founded in 2005. It is a top academic journal focusing on the field of metabolic biology under Cell Press (Impact Factor 30.9, JCR Q1 area). As a sister journal of "Cell", it focuses on cutting-edge research in the field of metabolic biology, covering the entire chain of research from molecular mechanisms to clinical applications. The journal is an important communication platform for researchers in the field of metabolic biology and is of great significance in promoting the understanding of metabolism-related disease mechanisms and the development of treatment strategies.
Click on the lower left to read the original text and access the original text link.
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202512973