
Recently, the team of Professor Zhang Jianxiang from the Army Medical University and Chongqing-Guangdong Pathological Science Research Center and the team of Professor Luo Gaoxing from the Army Medical University published a research paper titled "Engineering a macromolecular JAK inhibitor for treating acute inflammation and endotoxaemia" in the international journal Nature Biomedical Engineering.

Uncontrolled acute inflammation and persistent chronic inflammation are inextricably linked to the pathogenesis of many diseases, such as cytokine storm, acute pneumonia, sepsis, acute kidney injury (AKI), atherosclerosis, asthma, and neurodegenerative diseases. Therefore, anti-inflammation is crucial in the treatment of many diseases. The research team developed a new, efficient and safe macromolecule anti-inflammatory therapy - HPL. HPL is constructed by covalently linking polyethylene glycol (PEG) and luminol to a multivalent, hydrolyzable cyclic backbone (hexachlorocyclotriphosphazene, HCCP).
The conjugate can be easily synthesized with well-controlled structure and defined hydrolysis behavior. The team deeply explored the anti-inflammatory mechanism of HPL in macrophages and neutrophils. Experiments have shown that in the macrophage inflammation model stimulated by LPS, HPL can significantly inhibit the mRNA and protein expression of inflammatory factors such as TNF, IL-1β, IL-6, IL-8, MCP-1, and effectively inhibit macrophage migration. ; In the PMA-stimulated neutrophil model, HPL also dose-dependently downregulated the expression of inflammatory factors and significantly inhibited cell migration and activation. Notably, the effective dose of HPL is much lower than traditional anti-inflammatory drugs such as indomethacin and dexamethasone. In addition, further mechanism studies found that HPL exerts anti-inflammatory effects by inhibiting the IL-6/JAK2/STAT3 signaling pathway. Comprehensive verification of cellular uptake, signaling pathway inhibition and functional indicators lays the foundation for its highly effective anti-inflammatory effect in the body.
Based on the above in vitro data, the research team systematically evaluated the pharmacokinetics, target distribution and anti-inflammatory effect of HPL in various mouse inflammation models such as acute lung injury (ALI), acute kidney injury (AKI) and acute liver injury (ALF). In vivo fluorescence tracing experiments show that HPL micelles can continue to circulate within 12 hours after intravenous injection, and are preferentially enriched in damaged lungs, liver, kidneys and other inflammatory organs, and are mainly located in CD68+ macrophages and Ly6G+ neutrophils. Specifically, in the ALI model, HPL significantly reduced the lung wet-to-dry ratio, down-regulated the expression of multiple inflammatory factors, reduced the infiltration of neutrophils and macrophages, and effectively reduced inflammatory edema and tissue damage. ; In the AKI model, HPL not only improved renal function (lowered blood urea nitrogen and creatinine), but also significantly inhibited inflammatory factors and cell apoptosis. ; In the ALF model, HPL effectively reduced liver inflammation, improved liver function indicators, and inhibited neutrophil infiltration and MPO expression. These in vivo results consistently demonstrate that HPL exhibits superior efficacy to traditional anti-inflammatory drugs in a variety of acute inflammation models in a dose-dependent manner. In addition, thanks to its special structure, HPL can be used as a bioactive, inflammation-targeting, and inflammatory response drug carrier for site-specific delivery of hydrophobic drugs, thereby providing synergistic anti-inflammatory effects.
Furthermore, HPL demonstrated good safety in mice, even at doses five times greater than the therapeutic dose, and was hydrolyzable into biocompatible molecules in vitro. Together, these findings demonstrate that HPL, as a highly efficient, cost-effective, and safe novel JAK2 inhibitor, has great application prospects in the treatment of various inflammation-related diseases.
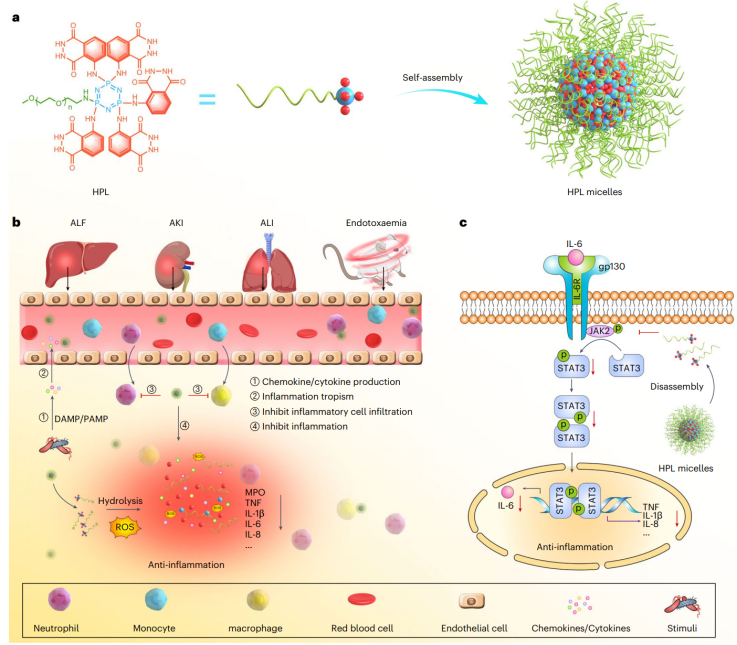
▲Schematic diagram of anti-inflammatory macromolecules and their derivative micelle therapy for the treatment of acute inflammatory diseases by regulating the JAK2/STAT3 signaling pathway to improve the pathological microenvironment.
"Nature Biomedical Engineering" is an authoritative international journal under the "Nature" series focusing on the field of biomedical engineering (impact factor 26.6, JCR Q1 area). It is published by Nature Portfolio and was founded in 2017. The journal focuses on cutting-edge research in the interdisciplinary field of biomedical engineering and publishes original results that deeply integrate engineering, materials science, biology and clinical medicine. It is an authoritative platform for researchers in this field to publish cutting-edge results.
Click on the lower left to read the original text and access the original text link.