
Recently, Professor Ding Yanqing’s team from Nanfang Hospital and Jinfeng Laboratory of Southern Medical University, Academician Bian Xiuwu’s team from Southwest Hospital of Army Medical University and Jinfeng Laboratory, and Professor Yu Weimiao’s team from the Singapore Agency for Science and Technology (A*STAR) published a paper titled “Cytological Classification Diagnosis for Thyroid Nodules via Multimodal Model Deep” in the international journal Advanced Science. Learning" research paper, successfully developed a new artificial intelligence model-AI-TFNA, which is expected to significantly improve the diagnostic accuracy and clinical management efficiency of thyroid nodules.
Thyroid nodules are a common endocrine system disease, affecting more than 60% of the general population worldwide, and are particularly common in women. Most thyroid nodules are asymptomatic, benign lesions, but 5% to 17% of cases are ultimately confirmed to be malignant. According to 2022 data released by the World Health Organization's International Agency for Research on Cancer, thyroid cancer has ranked as the seventh most common malignant tumor in the world, and its incidence is showing a rapid upward trend. This increase is mainly attributed to two factors: First, the actual increase in the incidence of thyroid cancer caused by environmental pollution and long-term ionizing radiation exposure ; Second, due to the sensitivity of diagnostic tools and advances in detection technology, more cases are being discovered. It is worth noting that while the incidence of thyroid cancer is rising rapidly, its mortality rate has remained at a low level. This difference is partly due to possible clinical misdiagnosis and over-treatment of thyroid nodules.
The research team successfully developed the AI-TFNA model, which innovatively combines deep learning and machine learning technologies and strictly follows the internationally accepted Bethesda Reporting System for Thyroid Cytopathology (TBSRTC) standards. It can provide an objective and reproducible AI-assisted tool for thyroid nodule cytology diagnosis, helping to reduce subjective differences and reduce the risk of misdiagnosis and over-treatment.
Highlight 1: Diverse data collection to enable the development and verification of robust models
This study collected a total of 20,803 thyroid liquid-based cytology samples from 7 medical centers in different regions of China for model training, validation, and testing. To take into account the variability encountered in real-world settings and to ensure the generalization ability of the model, each medical institution implemented different slide preparation and staining techniques and used different models of scanners to form full-field digital section images (WSI).
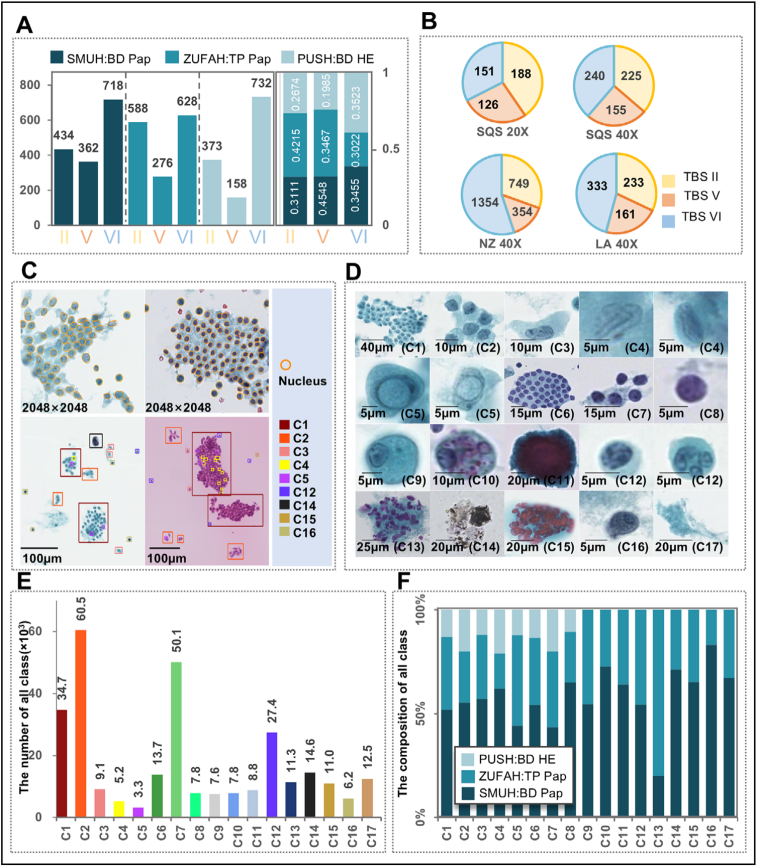
Figure 1 Data preparation and annotation.
Highlight 2: AI-TFNA improves pathologists’ diagnostic accuracy and efficiency
In the AI-TFNA auxiliary diagnostic experiment, the diagnostic accuracy of junior cytopathologists increased by 7.83%, and the diagnostic efficiency increased by approximately 1.93 times.; The average accuracy of senior cytopathologists increased by 2.68%, and their efficiency increased by approximately 2.04 times.
Highlight 3: Comprehensive internal and external validation confirms the diagnostic accuracy and reliability of AI-TFNA in different clinical settings
1. Evaluation of model performance: 108,517 WSIs from internal institutions and 2,153 WSIs from external institutions were used to test AI-TFNA in different slide preparation methods (BD SurePath, ThinPrep), staining techniques (Pap staining, HE staining) and different scanning methods (scanning equipment: Hamamatsu, Leica, Shengqiang, Jiangfeng, Una; Scanning times: 20, 40) performance in various clinical scenarios, the results show that AI-TFNA has strong generalization.
2. Evaluation of multimodal fusion models: Combining a BRAF mutation prediction model with a diagnostic model corrects misclassification.
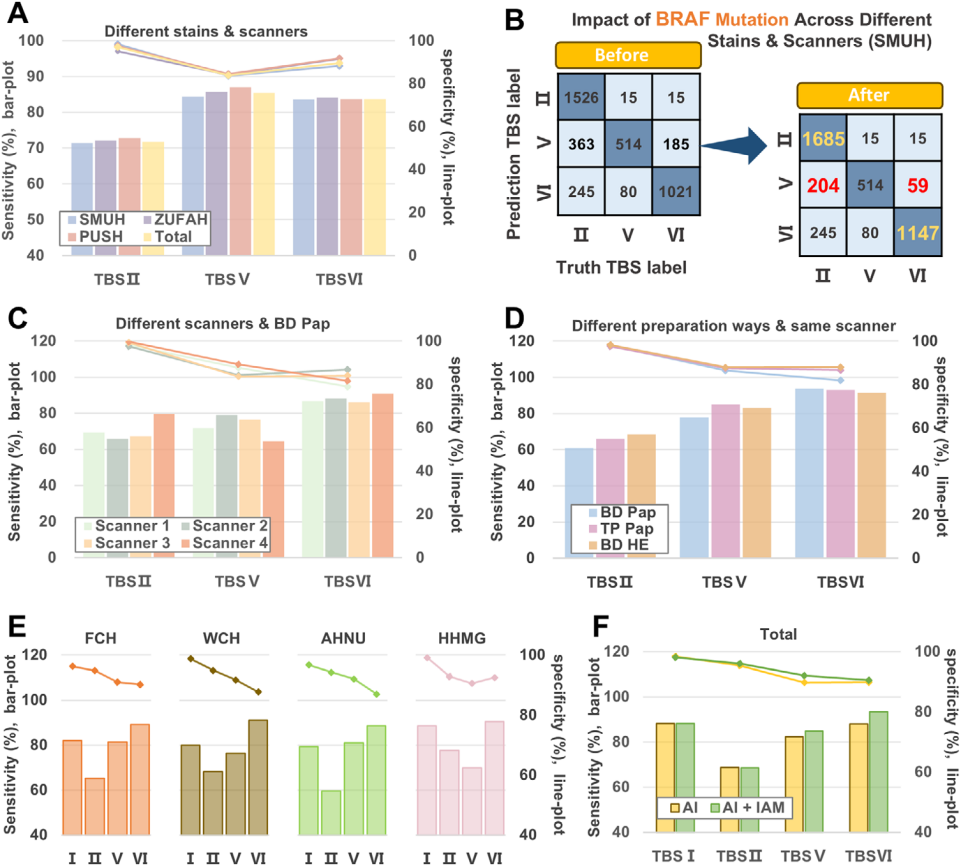
Figure 2 Diagnostic performance and clinical trials of AI-TFNA.
Highlight 4: Further improve the generalization ability of the model through image appearance transfer
This study used the Image Appearance Migration (IAM) method to verify on a multi-center data set, and the sensitivity increased by 1.90% and the specificity increased by 8.12%, which shows that even in the case of differences between different institutions, AI-TFNA has the potential to achieve stable performance and universal applicability.
AI-TFNA is designed in strict accordance with the diagnostic standards of TBSRTC. It uses artificial intelligence technology from the cell labeling stage to the final decision-making process, integrating cell number, cell nucleus/membrane morphology, texture and other related features to improve accuracy and clinical applicability. Among them, the VAN-tiny model component can effectively cope with complex clinical situations, accurately distinguish between benign and malignant thyroid follicular epithelial cells, and accurately distinguish between follicular epithelial cells and macrophages. It can be seen that AI-TFNA has important value in improving the accuracy and efficiency of clinical diagnosis of thyroid nodules, and is also expected to alleviate the current problems of uneven distribution of medical resources and serious shortage of pathologists in China.
"Advanced Science" is a well-known open source journal under Wiley Publishing House (impact factor 14.1, JCR Q1 area). It is committed to publishing top-level basic and applied research results in the fields of materials science, physics, chemistry, medicine, life sciences and engineering, and has high international academic influence.
Click on the lower left to read the original text and access the original text link.