
Lecture review
Lecture 1: Multi-element superstructure assembly & development of functional hydrogels and biomedical applications

Introduction to the speaker
Mao Xiang , Ph.D., Director/Associate Professor of the Engineering Teaching and Research Office, School of Biomedical Engineering, Chongqing Medical University, “Vice President of Scientific Research” of Chongqing Municipal Economic and Information Technology Commission, University & Enterprise, Member of the Graduate Education Supervision Group of Chongqing Medical University, and Degree Evaluation Expert of the Ministry of Education. He teaches courses such as "Medical Sensors" and "Biomaterials". Mainly engaged in the design and clinical medical application research of flexible biomaterials, hosted & participated in 6 national, provincial and ministerial level scientific research projects, and obtained 10 international patents and 15 domestic patents. Research areas mainly focus on: 1) Assembling functional nanostructures and their biomedical applications - combining chemical synthesis and molecular biology to research and develop ultra-nanostructures with new functional properties for potential clinical applications such as biomolecule detection, drug release, photothermal (kinetic) therapy, etc. ; 2) Design of metallo-like enzymes and applied research on enzymatic reactions - Based on the price-changing characteristics of multivalent metals, the solvent effect in chemical synthesis is used to prepare super-hydrophilic & ultra-small alloy nanoparticles to construct a simple, sensitive, and selective biosensor ; 3) Structural modification and simulation calculation of scaffold functional materials - combined with hydrogel scaffolds to form in-situ control to construct mesh scaffold materials with diverse structures, high biocompatibility, and high clinical applicability, analyze and establish quantum theoretical models, analyze the intrinsic mechanism of changing structure and performance, and realize normalized research on structure-performance-application-model calculation. He is currently a young editorial board member of European Cells & Materials and a guest editor of the International Journal of Molecular Science. He has published in high-level international journals such as JACS, ACS Nano, Bioactive Materials, Biomaterials, Advanced Healthcare Materials, Small, Nanoscale, Biomaterials Science, International Journal of Molecular Science, etc. He has published more than 70 high-level SCI academic papers.
Main content
Associate Professor Mao Xiang focused on core topics such as "Assembled Nano-Superstructures and Biomedical Applications", "Functional Hydrogel Materials and Wound Repair" and "Hybrid Peptide Hydrogels for Infectious Wound Healing". The lecture first systematically explained the core of the superstructure assembly strategy, focusing on how to accurately design and construct nanostructured primitives with different functions according to specific application requirements, and further solved the key scientific issue of how to controllably and efficiently assemble these nanoscale "building blocks" into macroscale functional materials. The report points out that developing new assembly strategies to break through the preparation bottleneck from "nano-units" to "macro-materials" is an important frontier direction for realizing the practical application of nanotechnology. Subsequently, the lecture focused on hydrogel materials, a research hotspot in the field of wound repair. The report fully affirms the huge potential of hydrogels due to their excellent biocompatibility, adjustable drug release capabilities and biomimetic extracellular matrix properties. It also clearly points out the three major bottlenecks faced by current technology: insufficient control of drug release kinetics, a single antibacterial mechanism that can easily lead to bacterial resistance, and the lack of effective means of active intervention in the wound microenvironment. To address the above challenges, the report focuses on a solution that integrates nanotechnology and materials science: that is, by organically combining inorganic nanomaterials with hydrogels (especially hybrid peptide hydrogels) to construct a composite functional hydrogel system. This strategy cleverly combines the unique properties of nanomaterials with the bionic environment of hydrogels, aiming to collaboratively solve the problems of infection control and tissue regeneration, especially providing new ideas for efficient healing of infected wounds. Ultimately, this cross-fusion of micro-nanotechnology and macro-tissue engineering opens up a promising path for promoting the innovation and clinical transformation of new biomedical technologies.
Lecture 2: Research on the fibrosis mechanism of self-assembled polypeptides and its application in neovascularization-related diseases
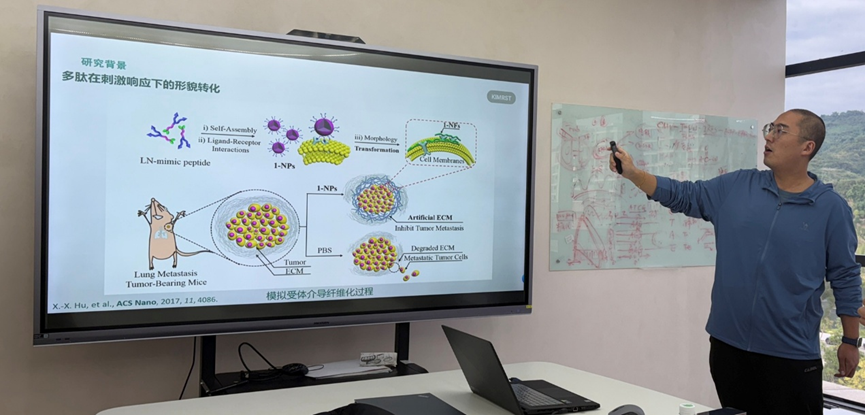
Introduction to the speaker
expand , Ph.D., Distinguished Associate Professor and Master Supervisor at the School of Biomedical Engineering, Chongqing Medical University. Mainly engaged in the assembly and fibrosis of polypeptides, and the application research of assembled polypeptides for disease diagnosis and treatment. He has hosted 1 National Natural Science Foundation of China project and published 10 academic papers as an author or co-author in journals such as Science Advances, ACS Nano, Biomaterials, Small, etc.
Main content
Associate Professor Zhang Kuang gave a systematic presentation on the research group's innovative exploration in the field of biomimetic peptide materials with the theme of "Research on the Mechanism of Polypeptide Molecules Simulating Fibronectin Fibrosis". The report points out that natural extracellular matrix proteins (such as fibronectin) can undergo fibrotic assembly under physiological or pathological stimulation, thereby supporting and fixing cells. Inspired by this natural process, the research team constructed a series of artificial peptide sequences that can simulate this key process through rational molecular design. The lecture focused on the in-depth study of its fibrosis mechanism. The reporter introduced in detail how to drive the controllable self-assembly of these synthetic polypeptides through receptor-mediated pathways, ultimately forming fibrous structures, revealing their dynamic evolution mechanism from molecules to supramolecular structures. In addition to elucidating the basic mechanism, the broad application prospects of this research are further demonstrated. The team successfully applied this biomimetic fibrotic peptide system to biomedical fields such as anti-corneal neovascularization and tumor embolization treatment. These application practices fully prove that through molecular simulation of natural protein functions, the developed peptide materials not only have biological functions similar to extracellular matrix, but also show great transformation potential in the treatment of major diseases, providing important ideas for the development of new biomaterials and treatment strategies.