
Recently, the team of Academician Bian Xiuwu from the First Affiliated Hospital of Army Medical University and Jinfeng Laboratory published a research paper titled "Ketogenic diet inhibits glioma progression by promoting gut microbiota-derivedbutyrate production" in Cancer Cell. It systematically revealed the mechanism of KD inhibiting glioma progression by reshaping intestinal flora and increasing butyric acid production, providing a scientific basis for the clinical application of KD's anti-glioma effect.

Ketogenic diet (KD) refers to a formula diet high in fat, low in carbohydrates, moderate in protein and other nutrients. It is generally believed that the human body enters a "ketogenic state" by reducing carbohydrate intake, forcing the body to "burn" fat to provide energy, which may have effects such as weight loss, intervening in the progression of epilepsy and type 2 diabetes. Glioma is the most common neuroepithelial tumor of the human brain. Its diffuse growth, extensive invasion, and recurrent drug resistance are the pathological basis of malignant biological behavior and seriously threaten human health and life. Therefore, it is of great significance to explore new targets and new intervention methods for glioma treatment. Experiments have shown that KD has a nutritional therapeutic effect on malignant glioma, but its clinical application is limited due to lack of sufficient evidence and unclear mechanism.
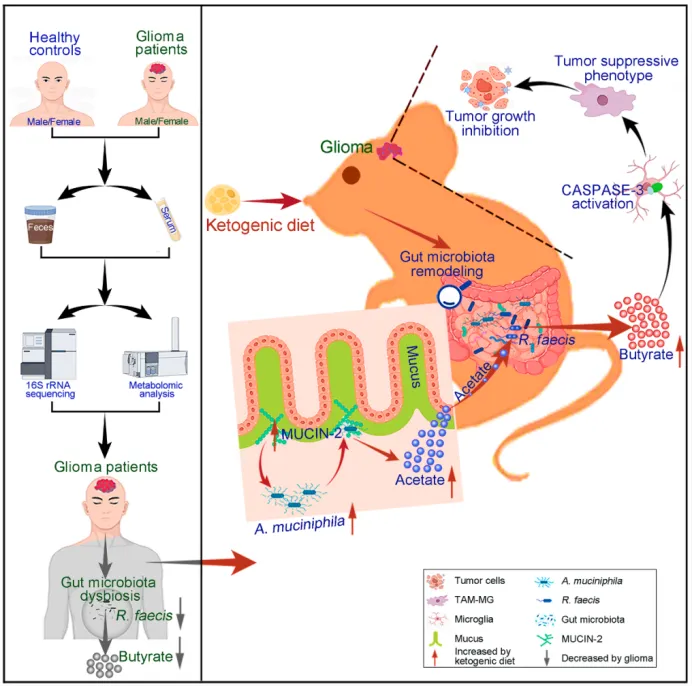
The team first found through population studies that the reduction of butyric acid-producing bacteria, especially R. faecis, and the decrease of butyric acid are typical characteristics of the intestinal microflora structure and metabolism of glioma patients. They then found in different mouse glioma models that treating tumor-bearing mice with antibiotics or sterilizing them promoted tumor growth and shortened survival time. Fecal microbiota transplantation (HC-FMT) of healthy controls or single bacterial transplantation of R. faecis inhibited the malignant progression of glioma in antibiotic-treated or germ-free mice. HC-FMT or R.faecis can significantly increase butyric acid levels in the feces, serum and tumors of tumor-bearing mice treated with germs or antibiotics, thereby attenuating the malignant progression of glioma tumors caused by antibiotic intervention or germ-free mice. ; It was also found that exogenous butyric acid supplementation had a similar effect. Using SnRNA-Seq and flow cytometry analysis, it was found that HC-FMT, R.faecis, and butyric acid induced a tumor suppressor phenotype of microglia (TAM-MG) in glioma, but had no obvious effect on monocyte-derived macrophages.
After the team further treated CX3CR1CreERT2/+:R26iDTR/+ mice with tamoxifen and diphtheria toxin to specifically eliminate TAM-MG, antibiotics, R.faecis or butyric acid had no significant effect on the progression of glioma. Mechanistic studies have found that R. faecis or butyric acid can improve the inactivation of caspase-3 in TAM-MG caused by germ-free mice or antibiotic intervention. Conditional knockout of caspase-3 in TAM-MG using CX3CR1CreERT2/+:CASP3flox/flox mice can significantly inhibit the tumor suppressor phenotype and tumor growth slowing effect of TAM-MG induced by R. faecis or butyric acid. In vitro experiments have also confirmed that butyric acid can significantly inhibit the release of IL6 and the reduction of iNOS in microglia caused by glioma cell supernatant. Silencing caspase-3 can reverse the above effects of butyric acid. The above results show that R. faecis is the core bacterium that regulates the progression of glioma. It specifically activates caspase-3 in microglia by increasing butyric acid production, inducing the anti-tumor phenotype of microglia, thereby inhibiting the progression of glioma.
On this basis, the team further found that KD can reshape the intestinal flora structure of glioma tumor-bearing mice, especially enrich A. muciniphila, increase butyric acid levels and activate caspase-3 in TAM-MG, promote the tumor suppressor phenotype of TAM-MG, thereby alleviating the malignant tumor progression of tumor-bearing mice. The anti-glioma effect of KD can be significantly inhibited by antibiotic treatment, conditional elimination of TAM-MG, or knockout of caspase-3 in TAM-MG. In addition, butyrate, A. muciniphila, R. faecis or A. muciniphila + R. faecis can alleviate the inhibitory effects of antibiotic intervention or germ-free mice on caspase-3 activation, IL6 reduction and iNOS increase in KD-induced TAM-MG. Finally, the authors found that KD increases the abundance of A. muciniphila by promoting the expression of mucin-2, thereby promoting the degradation of mucin-2 by A. muciniphila to produce acetic acid. ; Acetic acid provides sufficient substrate for R. faecis, ultimately prompting it to produce butyric acid.
Previous studies on KD's anti-glioma effects mostly focused on the direct effect of ketone bodies on tumor cells. In fact, tumor cells themselves can also utilize ketone bodies, which suggests that the anti-tumor "ketogenic effect" of KD may have a key mechanism other than the direct effect. In ancient times, there was the theory that "medicine and food come from the same source", and now there is the theory of "intestinal treatment of brain diseases". This study explains the therapeutic mechanism of ketogenic diet on malignant glioma from three levels: human population, animal and cell, that is, the key mechanism of the "gut flora-short chain fatty acid-brain microglia" axis in KD anti-glioma treatment. This research result not only provides a scientific basis for the anti-glioma effect of KD and the clinical application of the program, but also provides a new way to develop precision nutritional treatment strategies that target the intestinal flora.
"Cancer Cell" was founded in 2002 and is a comprehensive cancer research journal published by Cell Press. The journal aims to publish original cancer research papers, covering all aspects of cancer biology, pathology, molecular biology, genetics, and therapeutics. The latest impact factor is 48.8, ranking in the JCR Q1 area, and is a top journal in the field of oncology. The journal is committed to providing the highest level academic exchange platform for global scientists, clinicians and drug developers, and promoting knowledge dissemination and innovative development in the field of oncology.
Click on the lower left to read the original text and access the original text link.
https://doi.org/10.1016/j.ccel.2025.09.002