
recently, the team of academician bian xiuwu of southwest hospital of the army medical university and jinfeng laboratory of the jinfeng laboratory, together with professor feng hua's team, and professor wang yuhai of the wuxi no. 904 hospital, were inCancer Cellthe journal published a research paper titled "long-range cholinergic input promotes glioblastoma progression". revealed the important role and mechanism of remote cholinergic input in promoting the progression of glioblastoma (gbm). gbm can be integrated into neural circuits to promote its own progress through local and remote neural inputs, where remote cholinergic input is a cross-regionally conserved input type, and the released acetylcholine promotes gbm growth in a loop-dependent manner through muscarinic receptor chrm3. the acetylcholine m receptor inhibitor scopolamine can inhibit gbm growth, while the acetylcholinesterase inhibitor donepezil aggravates the condition. this discovery provides a new potential target for the treatment of gbm.

as the most deadly primary brain tumor, gbm has malignant progression closely related to the tumor microenvironment. in recent years, research has confirmed that glioma cells do not exist passively in brain tissue, but actively integrate into the neural circuit and promote their own growth through neuron-glioma interaction. during neurodevelopment, multiple neurotransmitters regulate neuronal proliferation and differentiation, while gbm cells express multiple neurotransmitter receptors, suggesting that they may achieve malignant progress by hijacking the neural modulation network. previous studies have found that local glutamategic synaptic connections are involved in the initiation and invasion of gbm, but the role of remote neural circuits and diverse neurotransmitter systems in it is still unclear. to this end, the research paper revolves around the following key content.
1. analysis of the whole-brain connection map of neuron-glioma cells and identification of neuron subtypes
in order to analyze the whole-brain connection network between neurons and glioma cells, the research team built a retrograde cross-single-synaptic tracing technology platform based on pseudoras virus (rabies virus). combined with fluorescence microscopic optical section tomography (fmost) and in situ hybridization technology, it systematically analyzed the distribution characteristics and subtypes of upstream neurons implanted in different brain regions and primary human glioma cells with different molecular types in the whole brain. the study not only confirmed the excitatory neuron connections around glioma cells that have been widely studied, but also found that glioma cells can establish extensive connections with distant neuromodulatory neurons distributed throughout the brain.
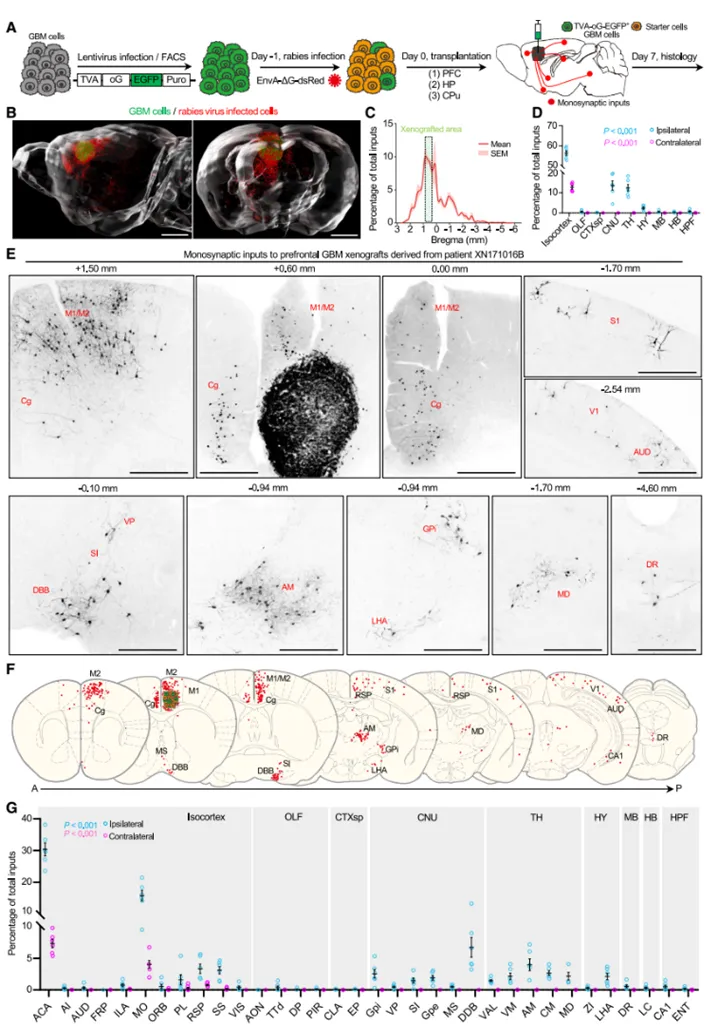
figure 1: whole-brain mapping of single synaptic connection neurons upstream of glioma cells
2. long-distance cholinergic neurons promote gbm through chrm3
the research team verified the structural and functional connections between long-distance cholinergic neurons and gbm cells through various methods. immuno-electron microscopy observed a symmetric synaptic structure formed between dbb-derived cholinergic terminals and transplanted gbm cells. optogenetics activates the projection of dbb cholinergic neurons in the prefrontal cortex, which induces stable calcium transients in gbm cells, an effect that can be completely blocked by cholinergic receptor antagonists. through gene editing and pharmacological intervention, it was found that cholinergic neurons release acetylcholine (ach) through synaptic release, activate the muscarinic acetylcholine receptor chrm3 on gbm cells, promoting tumor growth in a loop-specific manner. functional experiments show that selective ablation of dbb cholinergic neurons or knocking out chrm3 can significantly inhibit the proliferation and invasion of gbm and prolong the survival of tumor-bearing mice.
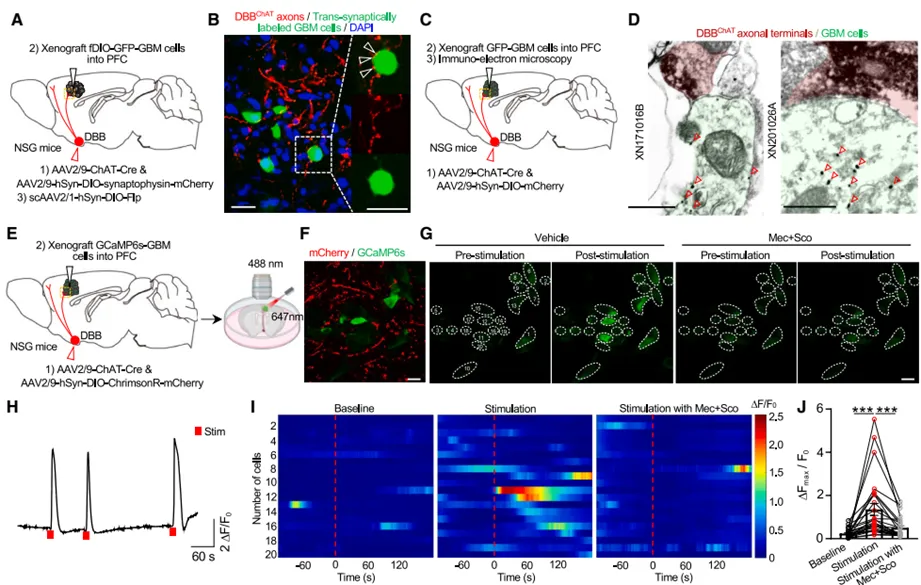
figure 2: synaptic connections and functional identification between glioma cells and upstream cholinergic neurons
3. pathway coordination and mechanism between cholinergic and glutamategic signals
the study found that cholinergic and glutamategic signals have both synergistic and functional differentiation in promoting gbm progression. calcium imaging showed that both neurotransmitters could increase the frequency and amplitude of spontaneous calcium transients in gbm cells, and combined stimulation produced a superposition effect. transcriptome analysis found that both signals activated the calcium signaling pathway and immediate early genes in the early stage (3 hours), but by the late stage (24 hours), cholinergic signal specifically regulates the expression of genes related to semaphorin-plexin signaling, mapk activation and neuroblast proliferation (jinfeng laboratory cell multiomics platform provides transcriptome sequencing and analysis services). in vivo experiments confirmed that blocking cholinergic and glutamategic receptors (chrm3 knockout combined with perampanel treatment) simultaneously produced stronger anti-tumor effects than a single intervention, significantly prolonging the survival of tumor-bearing mice.
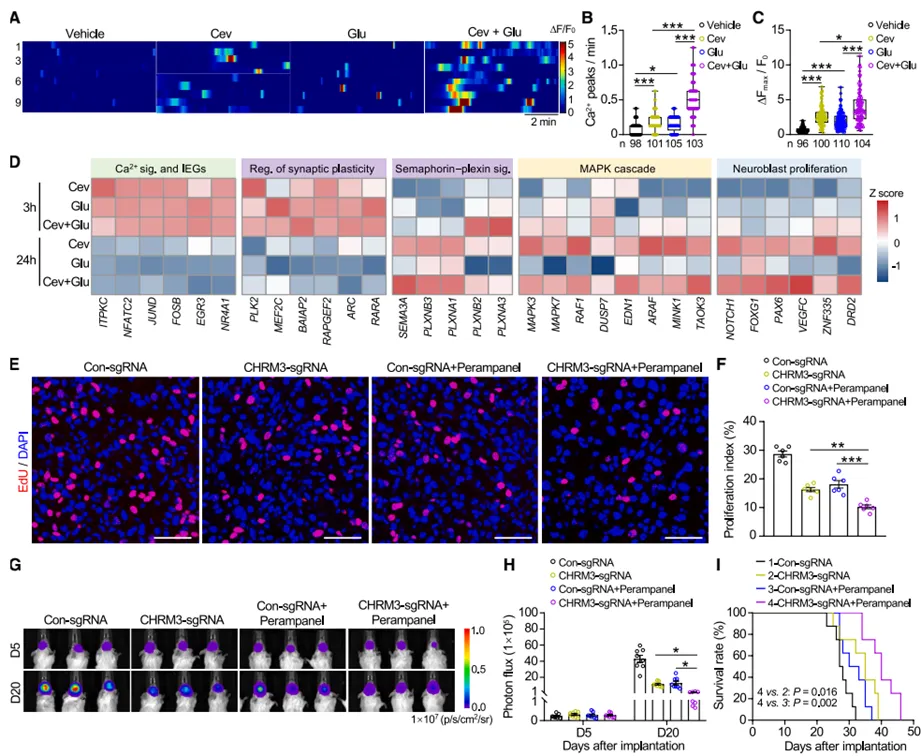
figure 3: differential effects and mechanisms of different neurotransmitters on gbm cells (glutamate vs. acetylcholine)
4. the clinical transformation potential of anticholiner drugs
this study has important clinical translational value. scopolamine, a commonly used anticholiner in clinical practice, can significantly inhibit the proliferation and invasion of gbm cells and prolong the survival of tumor-bearing mice; while donepezil, a cholinesterase inhibitor used to treat dementia, will aggravate the progression of gbm. the combined use of scopolamine and the standard chemotherapy drug temozolomide (tmz) produced a superimposed therapeutic effect, which increased the median survival of tumor-bearing mice by about twice as long as the control group. these findings suggest that targeting cholinergic signaling may become a new strategy for gbm treatment, and also warns the need to carefully evaluate the risk of use of drugs that improve central ach levels in gbm patients.
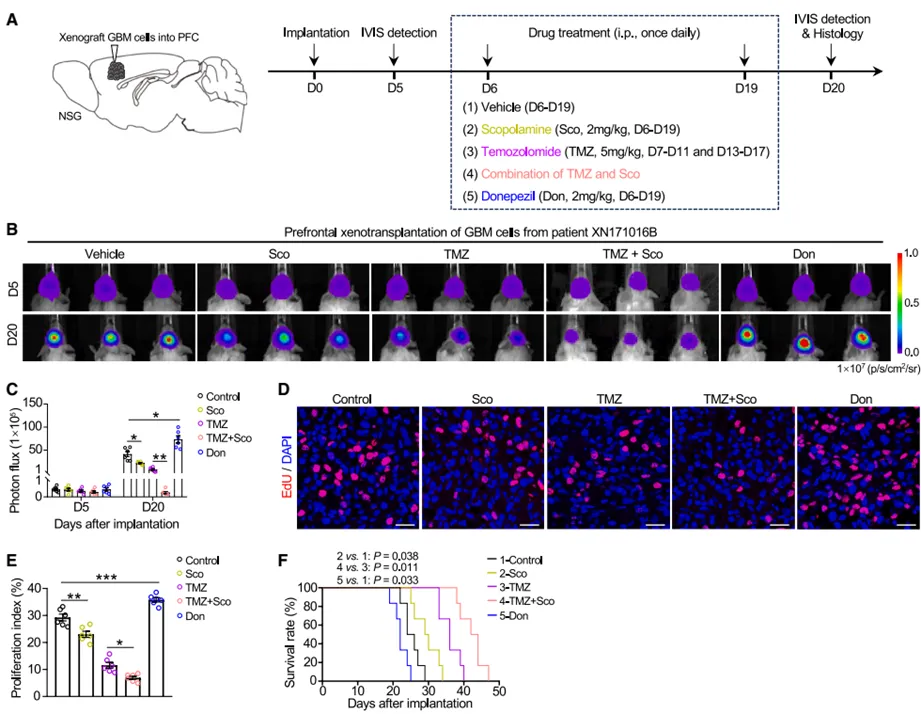
figure 4: common clinical drug intervention
summarize
this study systematically reveals a whole-brain map of neuron-gbm connections, confirming that remote cholinergic input is a key driver of gbm progression. its core conclusions include:
(1) different from existing studies, combined with multiple loop regulation technologies, the loop regulation mechanism of cholinergic neurons in the progress of gbm is revealed;
(2) comparative research and reveal the differential effects and mechanisms of different neurotransmitters on gbm cells (glutamate vs. acetylcholine);
(3) further proved the significance of clinical transformation, not only did it discover the potential of scopolamine to "newly used old drugs" to anti-gbm, but it also warned for the first time that acetylcholinesterase inhibitors may accelerate the progress of gbm, providing a new basis for clinical decision-making.
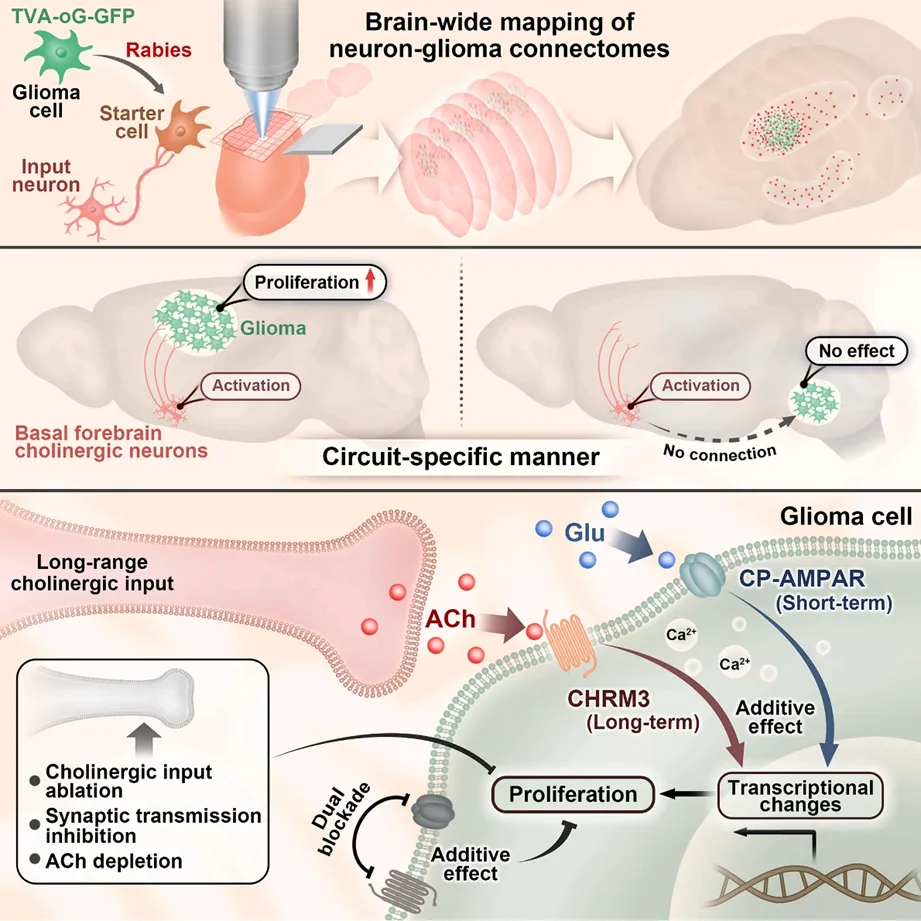
figure 5: article diagram
cancer cell was founded in 2002 and is a comprehensive cancer research journal published by cell press. the journal aims to publish original cancer research papers, covering research in various aspects such as cancer biology, pathology, molecular biology, genetics, and therapeutics. the latest impact factor is 44.5, ranking in the jcr q1 region, and is a top journal in the field of oncology. the journal is committed to providing the highest level of academic exchange platform for scientists, clinicians and drug r&d personnel around the world, and promoting the dissemination and innovative development of knowledge in the field of oncology.
click on the lower left to read the original text and access the original text link.
https://pubs.acs.org/doi/10.1021/acsnano.5c02492