
Research News | Professor Tian Gan’s team makes new progress in the field of biosensing based on plasmon nanoenzymes

Recently, Professor Tian Gan's team in Jinfeng Laboratory worked with Associate Professor He Ling of Chongqing University School of Pharmacy to publish a research paper titled "Triple-synergistic hollow AuAg@CeO2plasmonic nanozymes enable rapid alkaline phosphatase detection via smartphone-integrated dual-mode biosensing" in the international journal Journal of Colloid and Interface Science (TOP journal of the Chinese Academy of Sciences, impact factor: 9.7). The research intern of the scientific research team, Tan Juan, is the first author of the paper, and Jinfeng Laboratory is the first communication unit of the paper.
Alkaline phosphatase (ALP) is an important hydrolase widely distributed in human tissues (such as the liver, bone, kidney and intestine). Its activity level is closely related to hepatobiliary diseases (such as cholestasis, hepatitis and cirrhosis) and bone diseases (such as osteosofolactivity and Paget’s disease), and is a key biomarker in clinical diagnosis. However, traditional ALP detection methods usually rely on complex instruments and cumbersome operations, and have limitations such as insufficient sensitivity and susceptibility to sample matrix interference, making it difficult to meet the needs of large-scale screening and real-time monitoring. Therefore, it is of great significance to develop a fast, cost-effective and ultra-sensitive ALP measurement sensing strategy.
This study designed and synthesized a near-infrared light-responsive AuAg@CeO2 plasmon nanoenzyme. Based on its plasmon-enhanced peroxidase-like activity, a new method for detecting the content of disease markers ALP was constructed and applied to the detection of clinical serum and urine samples. The hollow nanostructure enhances near-infrared photon capture by multi-scale light scattering, while the AuAg-CeO2 heterojunction enables directional charge transfer. ALP-mediated ascorbic acid generation triggers cascade mechanisms: (1) plasma thermal electron regeneration CeO2 oxygen vacancies to optimize H2O2 adsorption; (2) photothermal activation promotes H2O2 dissociation; (3) local surface plasmon resonance (LSPR) accelerates interface electron kinetics. This synergistic effect significantly improves its peroxidase-like activity, and it can achieve nanomolar ALP detection sensitivity in just 10 minutes - a 100-fold increase compared to commercial kits. Integrated colorimetric dual-mode analysis based on smartphones can achieve cross-verification of ALP quantification (matrix interference <10%), and human sample verification shows that the recovery rate is 94.8-105.4%. By analyzing the structure-LSPR-activity association, this study has created an adaptive nanoenzyme design and intelligent diagnosis platform for precision medicine, making up for the key technical gaps in real-time detection and early disease monitoring.
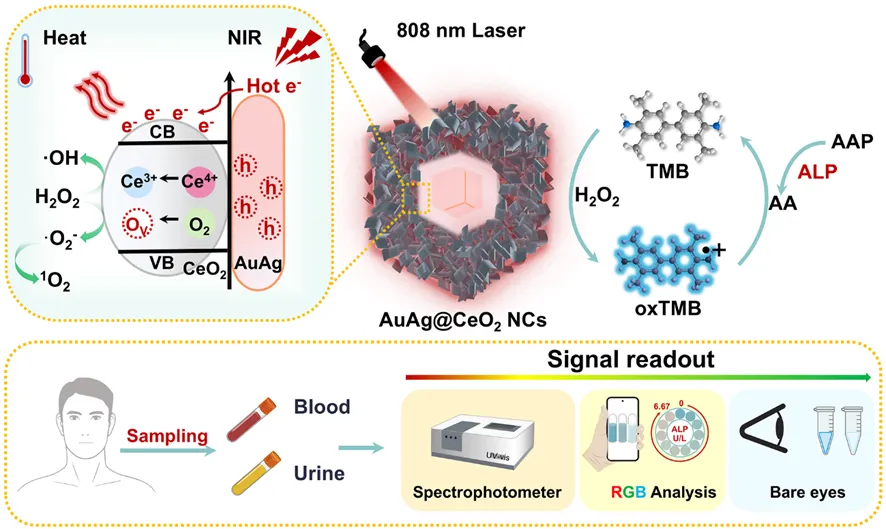
▲The mechanism of the enhancement of peroxidase activity of AuAg@CeO2 nanoenzymes under near-infrared light excitation and its schematic diagram of its visual detection probe as an ALP
Journal of Colloid and Interface Science Published by Elsevier Press, it focuses on the fields of colloidal science and interface science, covering multiple disciplines such as nanomaterials, surface chemistry, biocolloids, and dispersed systems. The content takes into account basic theoretical research and applied innovation (such as interface phenomena in energy, environment, and biomedicine). The latest impact factor of the journal is about 9.7, and it is Q1 in the JCR area. The latest upgraded version of the major materials science of the Chinese Academy of Sciences is located in District 1, and is an authoritative platform for scientific researchers in this field to publish cutting-edge results.
Click on the lower left to read the original text and access the original text link.
- About Us
-
Research Platform
- Major Disease Sample Database
- Innovative Drug Verification And Transformation Platform
- Experimental Animal Center
- Life And Health Future Laboratory
- Biomedical Imaging Platform
- Cell Multi-Omics Platform
- Pathology Technology Platform
- Bioinformatics Research And Application Center
- Jinfeng Pathology Precision Diagnosis Center
- Research Team
- Information Center
- Join Us


 渝公网安备50009802002274
渝公网安备50009802002274